Local News
Researchers at the University of Rochester identify key cells behind poor tendon healing and discover a method to retrain them for better recovery outcomes
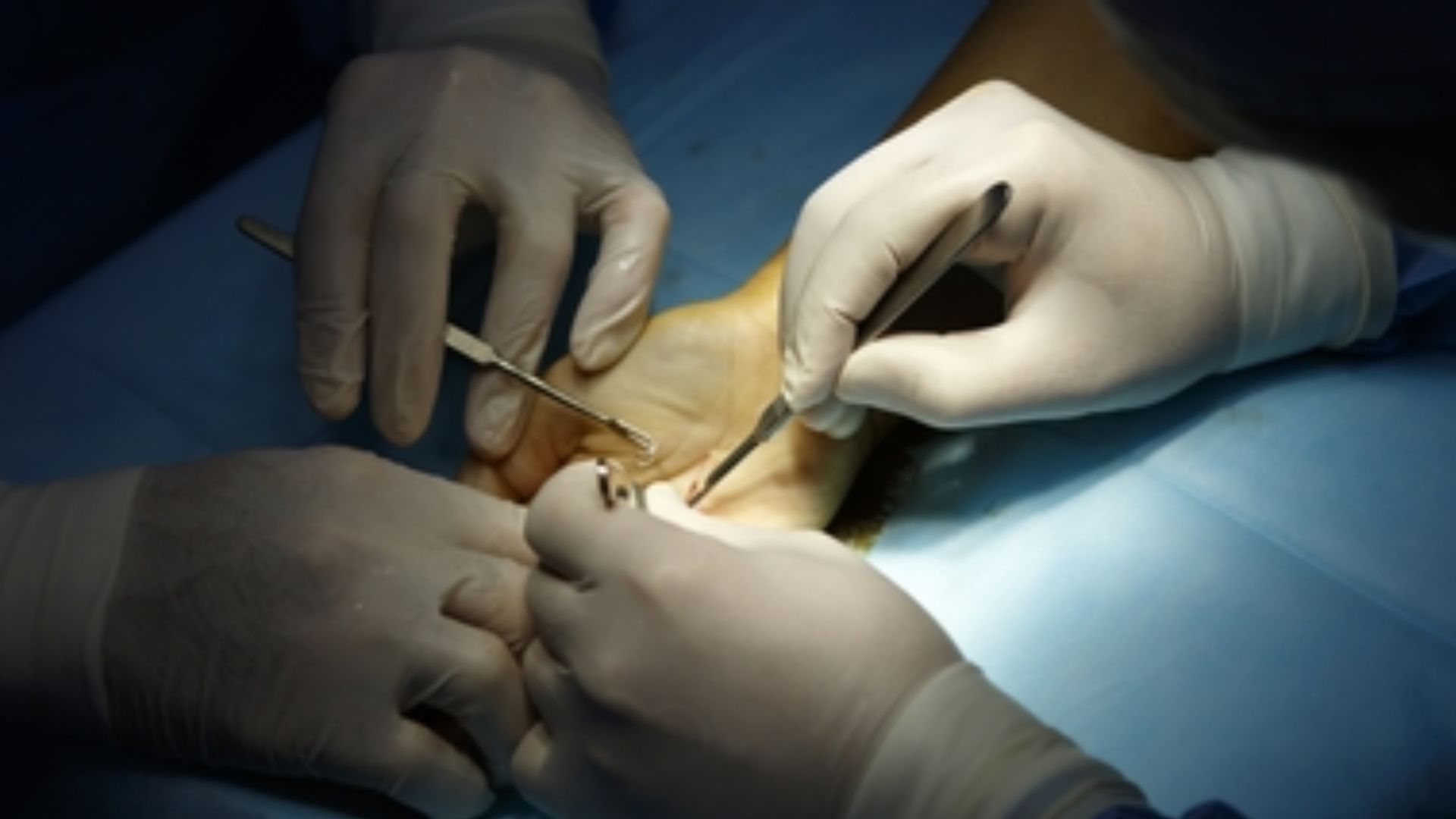
Rochester, New York – In a groundbreaking advancement for musculoskeletal medicine, researchers at the University of Rochester have uncovered a previously elusive culprit behind poor tendon healing — and for the first time, they’ve found a way to control it. Their findings, recently published in Nature Communications, spotlight the role of epitenon cells, a thin layer of cells that wrap around tendons, and how they can either help or hinder recovery after surgical repair.
This discovery is a pivotal moment in the field of tendon biology — a topic that, until recently, received little attention from researchers despite the enormous impact tendon injuries have on public health. According to estimates, flexor tendon injuries in the hand alone account for around 300,000 surgeries annually in the U.S., resulting in more than $400 million in healthcare costs. Even after surgery, many patients are left with limited mobility and pain due to excessive scar tissue. Nearly one in four will require a second operation just to remove the buildup.
Yet despite these sobering statistics, there are still no targeted post-surgical treatments to improve how tendons heal, other than physical therapy.
A Fresh Look at a Complex, Often Misunderstood Structure
For decades, tendons were thought of as relatively simple anatomical structures — essentially strong, stringy ropes connecting muscle to bone. But researchers at Rochester’s Center for Musculoskeletal Research (CMSR) are leading a scientific shift. Their work reveals that tendons are anything but simple. They are intricately layered and filled with diverse populations of cells that behave differently across regions and stages of healing.
Read also: State Police take Nassau man into custody after a string of burglary cases across multiple
“One of the challenges in tendon is being able to target all these different cell populations,” explained Anne Nichols, PhD, assistant professor of Orthopaedics and the study’s lead author. “Tendons in general heal poorly, but not uniformly. In different regions of the healing tendon, you’ll see different types of fibrosis develop. An effective therapy requires targeting these specific areas in different ways to modulate the healing process.”
Her team homed in on one key group: epitenon cells. These cells line the outer surface of tendons and had previously been overlooked in most research, which tended to focus on tenocytes — the cells that maintain the tendon’s basic structure. The Rochester scientists believed the epitenon played a bigger role than previously thought, particularly in how scar tissue forms.
Mapping the Role of Epitenon Cells
To unlock the secrets of these cells, Nichols and her team used cutting-edge tools like single-cell RNA sequencing and genetic lineage tracing. These techniques allowed them to follow individual epitenon cells in mice and observe their movement and behavior after a tendon was injured and surgically repaired.
They discovered something surprising: epitenon cells don’t all behave the same way. A small portion followed the tenocytes into the core of the tendon injury, helping to form new tissue and bridge the gap — a good thing. But a much larger group took a different path, migrating to the edges of the injury and building a thick capsule of scar tissue that restricted movement and complicated healing.
“We know there is a fibrotic burst of activity in an injured tendon between days 7 and 10 post-injury; we wanted to target pro-fibrotic epitenon cells in that time window to see how removing them from the injury site impacts healing,” said Nichols.
That’s exactly what they did. Using a genetically encoded toxin to eliminate only the epitenon-derived cells during this narrow window, the researchers observed a dramatic improvement in healing outcomes in mice. The tendons regained more natural range of motion, and scar tissue formation was significantly reduced — without weakening the tendon’s strength.
A Human Connection
Mouse models are essential in biomedical research, but the ultimate goal is to translate findings into treatments for people. To see if their results might hold up in humans, the team compared their mouse data to samples taken from injured human tendons. What they found was promising.
“The cells we identified as problematic for forming scar tissue in a mouse also exist in the human scar, which suggest they play a similar scar-forming role in humans following tendon injury,” Nichols said. “In theory, if we were able to target these cells in humans, we could get the same outcome in them as we could from a beneficial treatment in mice.”
The resemblance between the mouse and human cell populations supports the idea that this line of research could lead to new therapies that limit post-surgical complications and reduce the need for follow-up operations.
A New Era in Tendon Research
For decades, the biology of tendons remained in the background of musculoskeletal research. Bones and muscles took center stage, while tendons were seen as passive tissues — useful, but not particularly interesting from a cellular standpoint. That’s rapidly changing, thanks to work from CMSR and a small number of other global research centers.
“The epitenon contains a cell population that we knew was important, but could not target,” said Nichols. Now, not only have they figured out how to find these cells — they’ve demonstrated how to control their impact on healing.
The implications stretch beyond hand injuries. Tendon damage occurs in a wide range of contexts: from rotator cuff tears to Achilles tendon ruptures to sports injuries and age-related degeneration. Understanding how to modulate healing could improve outcomes for millions of people, particularly those whose injuries currently result in long-term disability or repeated surgeries.
Next Steps Toward Treatment
Although the study’s results are preliminary and focused on mice, they open a clear avenue for developing drugs or gene therapies that can be applied during a specific healing window to minimize scarring and improve recovery. Timing and location are critical — as the team discovered, removing too many of the wrong cells or acting at the wrong stage of healing could have unintended consequences.
Still, the progress is significant. This research redefines our understanding of tendon recovery and lays the foundation for targeted interventions that were once thought impossible.
The study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), a division of the NIH. Alongside Nichols, contributors included Lauren Benoodt, Emmanuella Adjei-Sowah, Kyle Jerreld, Alexander Kollar, Constantinos Ketonis, and Alayna E. Loiselle — all researchers committed to revealing the hidden complexity of the body’s most underrated tissue.
As Nichols and her team continue to trace the healing journey of individual cells, their work promises to offer new hope for patients facing painful recoveries and uncertain outcomes. With further research, the idea of retraining cells for better healing may no longer be science fiction — but standard care.

-

 Local News12 months ago
Local News12 months agoNew ALDI store close to Rochester to begin construction in late 2025 or early 2026
-

 Local News12 months ago
Local News12 months agoRochester Lilac Festival announces exciting 127th edition headliners
-

 Local News10 months ago
Local News10 months agoCounty Executive Adam Bello and members of the county legislature celebrate exceptional young leaders and advocates at the 2025 Monroe County Youth Awards
-

 Local News10 months ago
Local News10 months agoThe 2025 Public Market Food Truck Rodeo series will begin this Wednesday with live music by the Royal Bromleys